Background: Azacytidine is one of the hypomethylating agents (HMAs) for the treatment of higher-risk myelodysplastic syndromes (MDS). However, clinical outcomes of patients treated with azacytidine monotherapy were far from satisfactory with overall response rate (ORR) of 41%-59% and overall survival (OS) of 15-18 months. Selinexor is an oral, first-in-class selective inhibitor of nuclear export compound that inhibits exportin 1 (XPO1). Preclinical studies have shown that inhibition of XPO1 causes nuclear accumulation of p53 and disruption of NF-κB signaling, both relevant targets for myelodysplastic syndromes. Prior study reported an ORR of 26% and mOS of 8.7 months with selinexor in HMAs-refractory MDS patients. We therefore aimed to assess the safety and efficacy of selinexor sequential azacytidine in newly diagnosed patients with MDS-EB1 or EB2.
Methods: The effectiveness of simultaneous and sequential treatment of azacytidine and selinexor was measured by cell death rate, cell cycle and colony formation rate in vitro. Western Blot was performed to detect the sublocation of p53. MOLM-13-luciferase xenograft murine model was established to verify the synergetic effect in vivo. Based on the experiments, we conducted a single-center, single-arm, phase Ib/II trial, which included newly diagnosed patients 18 years or older with MDS-EB1 or EB2. Eligible patients received 2-4 weeks long cycles of oral selinexor (40mg qw for 4 weeks; 40mg biw for 2 weeks; 60mg qw for 4 weeks). The primary endpoints were to find the recommended phase 2 dose (RP2D) and maximum tolerated dose (MTD). The secondary endpoint was ORR including complete remission (CR), partial remission (PR), marrow complete remission (mCR), and hematological improvement (HI). This trial was registered with the Chinese Clinical Trial Registry (#ChiCTR2200065167).
Results: First we treated three AML cell lines: AML-OCI2, THP1, MV4-11 and three MDS cell lines: SKM-1, MOLM-13, MDSL with azacitidine and selinexor simultaneously or sequentially. Sequential treatment showed stronger synergetic effect (Figure 1A) and reduced colony formation by 90% in SKM-1 and MOLM-13 (Figure 1B). Cell cycle arrest were induced and the percentage of Annexin V cells obviously increased with sequential treatment (Figure 1C). Sequential treatment decreased leukemia burden and prolonged survival of MOLM-13-luciferase xenograft murine model (Figure 1D, E). Mechanistically, pretreatment of azacitidine on SKM-1 and MOLM-13 upregulated the expression level of p53, and selinexor led to massive nuclear accumulation of p53, resulted in better vulnerability lethal to MDS cells (Figure 1F).
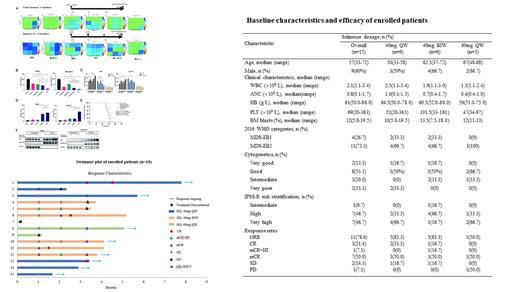
Secondly, 15 patients were enrolled in our trial with a median age of 57. 26.7% of patients were MDS-EB1, 73.3% were EB2. 93.3% of patients were high or very high risk by the Revised International Prognositic Scoring System (IPSS-R) (see the Table). At Phase Ib, the following selinexor dosages were administered: 40mg qw (n=3), 40mg biw (n=3) and 60mg qw (n=3). One patient in the 60mg qw group discontinued treatment because of nausea, vomiting, fatigue and Herpes simplex virus infection. Subsequently, in order to find RP2D, an extension cohort of 3 additional patients were treated with 40mg qw and 40mg biw, respectively. Therefore, 14 patients were evaluable for efficacy assessment. ORR was 78.6% in 11 patients including CR in 3 patients (21.4%); mCR+HI in 1 patient (7.1%); and mCR in 7 patients (50.0%) (see the swimmer plot). Especially, among the TP53 mutated patients (n=3), one achieved CR, the other two achieved mCR. The common adverse events were neutropenia (93.3%), anemia (86.7%), thrombocytopenia (80.0%), fatigue (53.3%), nausea (26.7%), dizziness (26.7%), vomiting (20.0%) and decreased appetite (20.0%). There were no treatment related deaths.
Conclusion: We verified the activity of selinexor sequential azacytidine in MDS both in vitro and in vivo. The treatment showed with encouraging efficacy in newly diagnosed patients with MDS-EB1 or EB2. Adverse events were manageable with supportive care implementation. Enrollment is ongoing to assess the efficacy and safety of this sequential therapy, which may be a new treatment option for EB subjects.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal